|
|
Breaking news
[Back to archive]
The latest results achieved by the project consortium.
Covert shift of attention modulates the ongoing neural activity in a reaching area of the macaque dorsomedial visual stream
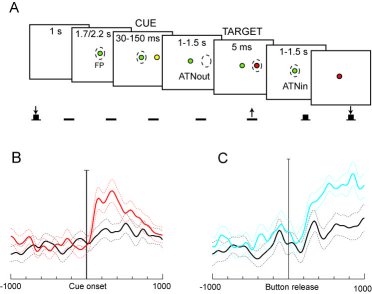
A) The task performed by the animal; B) Population data showing V6A responses to covertly attending in the preferred (red line) and in the opposite (black line) directions. C) Population data with responses back to the fixation point while waiting for its change in color; preferred, blue line, opposite, black line.
Overt deployment of attention is seen in directing saccadic eye movements to salient or task-relevant parts of the scene, but attention can also be deployed covertly, without any visible motor activity. Covert orientation of attention is done by internally modulating the processing of information in visual cortical maps, and by selecting parts of the scene to receive increased processing resources. The selection of the part of the scene to receive attention, i.e. the control of the focus of attention, is driven by the saliency of the stimuli and by the requirements of the task that is currently performed. It is closely related to the motor actions that are to be performed on the selected targets, in particular to the preparation of eye movements.
However, the link between attention and goal-directed motor actions is not confined to the eye movements. Also the preparation of reaching movements is paralleled by a shift of attention to the goal of the reach (Castiello, 1996; Deubel et al., 1998). It has also been demonstrated that attentional selection for simultaneous reaching and eye movements to different targets shows some degree of independence between the two, such that both goals can receive processing benefits (Jonikaitis and Deubel, 2009). Thus, one might expect that, similarly to oculomotor areas that provide signals for overt and covert shifts of attention, also cortical areas that are involved in the generation of arm movements may contribute to attentional shifts.
In this research we checked whether the ongoing activity of single cells in V6A (a visuomotor area of the dorso-medial visual stream involved in coding reaching movements to targets in space (Galletti et al., 2003; Fattori et al., 2005)) was influenced by the shifts of covert attention without any concurrent shift of the direction of gaze. We performed extracellular recordings on single cells of area V6A of 2 Macaca Fascicularis. The task performed by the animal is summarized in figure 1A. Animals were trained to fixate in the straight-ahead position on a light-emitting diode (LED), in darkness, while pressing a button located outside their field of view. The monkeys were trained to maintain the gaze in the straight-ahead position all throughout the trial. Their fixation was checked using an infrared oculometer. The animals had to detect a target (5 ms- red flash) in one out of several peripheral (eccentricity = 15°) positions and to respond to it by releasing the button. The target position was cued by a yellow flash (30-150 ms) presented in the same location as the target but preceding the target onset by a variable interval of 1000-1500 ms. The cue prompted the monkeys to covertly displace attention towards it. After target detection, the monkeys had to shift the attention back towards the straight-ahead position because it had to detect a change in color of the fixation LED, and to report detection by pressing the button again. We calculated the average discharge rate of each cell during fixation before the cue onset (baseline activity) and during different time epochs after the cue onset. Out of 92 tested cells, 46% were modulated by this task (two-way ANOVA [factors: epoch x target position] and multiple comparisons with Bonferroni correction, P < 0.05). Of these task-related cells, 61% were tuned during epochs starting well after the cue onset (200 - 500 ms after cue onset). Fig. 1B shows the discharge of all tested V6A cells activated when the attention was shifted toward the periphery, after the presentation of the cue in that spatial location. For comparison, the neural discharge of the same population is also shown when the attention is shifted in the opposite direction. It is evident a sustained activity of the cells that lasts more than 500 ms, that is well beyond the cue visual stimulation. We suggest that this sustained activity is the result of the displacement of the spotlight of attention.
Moreover, 76% of task-related cells were tuned when attention was shifted back to the straight-ahead position after target detection. This modulation cannot be ascribed to any appearance of a visual stimulus, as the fixation point was continuously present and continuously fixated by the animal. Population discharges in this epoch are summarized in Fig. 1C.
These data show that the medial posterior parietal area V6A contains neurons that reflect internal orientation mechanisms. These neurons can locate or select peripheral targets to be reached and grasped by the animal.
References
Castiello U (1996) Grasping a fruit: selection for action. J Exp Psychol Hum Percept Perform 22:582-603.
Deubel H, Schneider WX, Paproppa I (1998) Selective dorsal and ventral processing: Evidence for a common attentional mechanism in reaching and perception. VisCogn 5:81-107.
Fattori P, Kutz DF, Breveglieri R, Marzocchi N, Galletti C (2005) Spatial tuning of reaching activity in the medial parieto-occipital cortex (area V6A) of macaque monkey. Eur J Neurosci 22:956-972.
Galletti C, Kutz DF, Gamberini M, Breveglieri R, Fattori P (2003) Role of the medial parieto-occipital cortex in the control of reaching and grasping movements. Exp Brain Res 153:158-170.
Jonikaitis D, Deubel H (2009) Split attention during simultaneous eye and hand movements. Perception ECVP Abstract Supplement 38:158.
Claudio Galletti(1), Rossella Breveglieri(1), Markus Lappe(2), Annalisa Bosco(1),
Marco Ciavarro(1), Patrizia Fattori(1)
Department of Human and General Physiology
University of Bologna (1)
Department of Psychology
University of Münster (2)
|
|
|

|

WEBMASTER: Agostino Gibaldi (UG)
|
|